
Electrolysis:
CONTENTS
REVISION OF IONIC SUBSTANCES
Atoms react with each other to form full outer shells of electrons. The easiest way for metal atoms to get a full outer shell is to lose electrons. The easiest way for non-metal atoms to get a full outer shell is to gain electrons. When a metal reacts with a non-metal, electrons are transferred from the metal atoms to the non-metal atoms, forming ions.
Metal atoms lose electrons, forming positive ions, and non-metal atoms gain electrons, forming negative ions. The compound formed is made of ions, which are strongly attracted to each other because of their opposite charges. This is called ionic bonding.
If you want to learn more about ionic bonding, see Ionic Bonding
WHAT IS ELECTROLYSIS?
Solutions that conduct electricity are called electrolytes. When a direct electric current is passed through an electrolyte, chemcial changes occur (reactions occur at the electrodes). This is known as electrolysis.
DEFINITION: Electrolysis
Chemical change caused by passing an electric current through an electrolyte.
When solids conduct, they do so without chemical change occurring (e.g. metals and graphite). They are called conductors. Solids that do not conduct electricity are called insulators.
ELECTROLYSIS CELL DIAGRAM
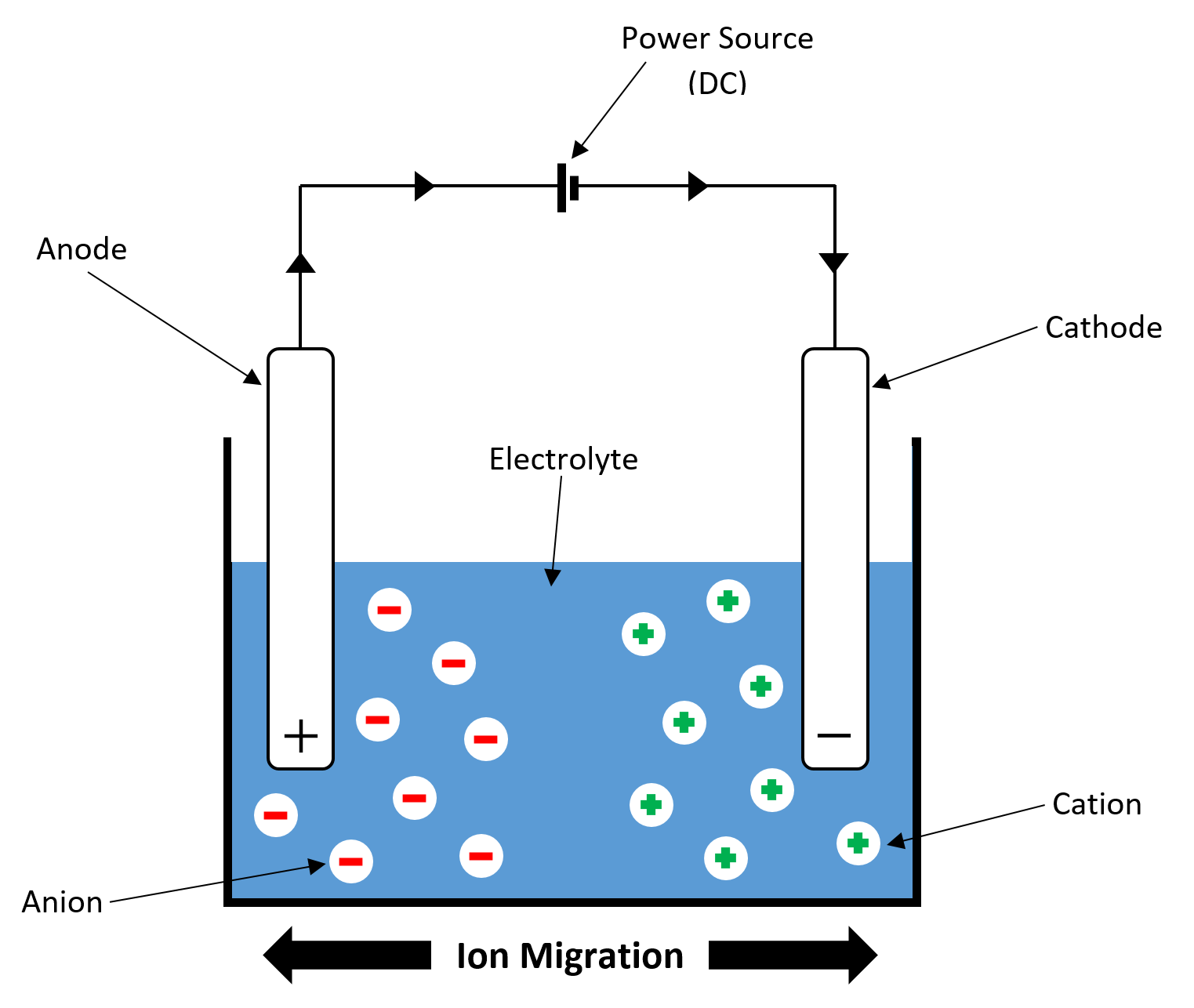
DEFINITION: Electrode
A solid electrical conductor that connects the power source to the electrolyte. Graphite is a common electrode material as it conducts electricity and is chemically inert.
DEFINITION: Anode
The positive electrode.
DEFINITION: Cathode
The negative electrode.
DEFINITION: Anion
A negatively charged ion. Anions are attracted to the anode (positive electrode) during electrolysis.
DEFINITION: Cation
A positively charged ion. Cations are attracted to the cathode (negative electrode) during electrolysis.
DEFINITION: Electrolyte
A solution or molten substance that conducts electricity. When a current is passed through an electrolyte, electrolysis takes place.
DEFINITION: Ion Migration
The movement of ions from the electrolyte to the electrodes (anode and cathode) when an electrical current is passed through it.
ELECTROLYSIS OF MELTS
In melts, ions migrate to the oppositely charged electrode. At the cathode, the positive ions are reduced as they gain electrons, forming the metal. At the anode, the negative ions are oxidised as they lose electrons, forming non-metals in their elemental form.
See the example for lead bromide (PbBr2) below:
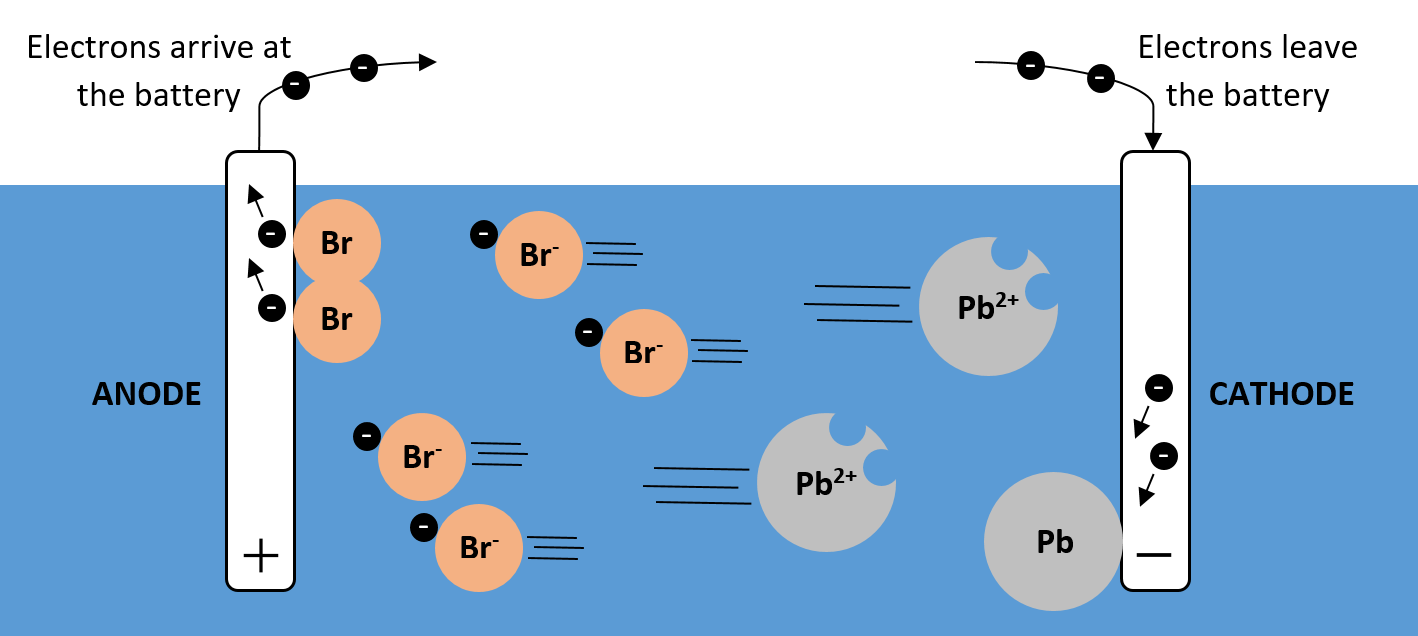
HALF EQUATIONS
Half equations show the reactions occurring at each of the electrodes, and indicate which product is formed.
e.g. For the above example of the electrolysis of lead bromide:
Pb2+ + 2e- Pb
At the cathode, lead ions, Pb2+, gain two electrons. They form lead atoms.
2Br- Br2 + 2e-
At the anode, two bromide ions, Br-, each lose their extra electron. They form Br2 molecules.
REDOX AS ELECTRON TRANSFER (OILRIG)
Electrolysis is a REDOX reaction. Oxidation occurs at the anode and reduction occurs at the cathode.
DEFINITION: REDOX Reaction
A reaction in which both oxidation and reduction take place.
Oxidation is a loss of electrons. Reduction is a gain of electrons.
You can remember this with the mnemonic OILRIG: Oxidation Is Loss. Reduction Is Gain.
ELECTROLYSIS OF AQUEOUS SOLUTIONS
When electrolysis is carried out on melts, there is usually only one type of cation and one type of anion present, so the products are simple to predict. However, when ionic substances are dissolved in water, the ions from the water (H+ and OH-) are also present, so there is more than one cation and more than one anion. This makes predicting the products of electrolysis more difficult.
Rules for Predicting the Products of the Electrolysis of Solutions:
At the cathode:
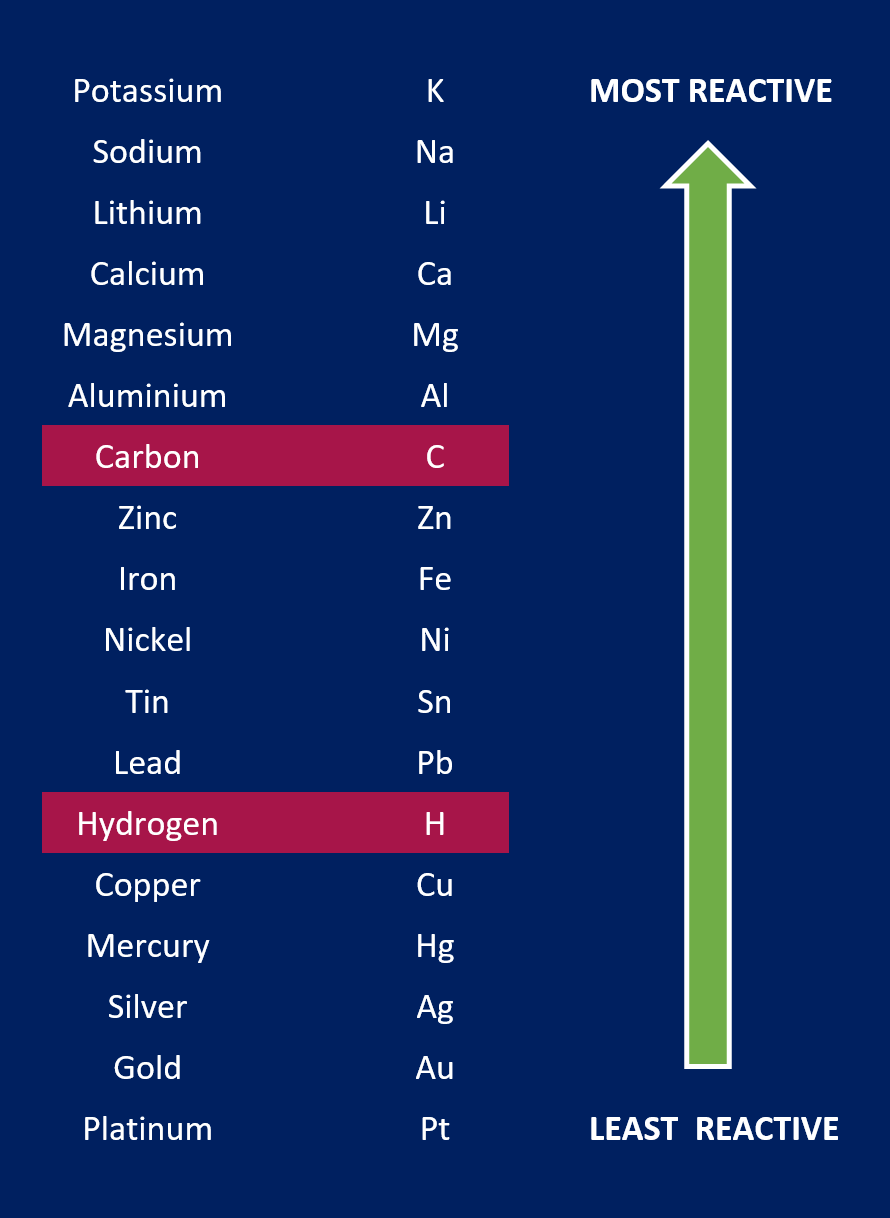
Whether hydrogen or the metal is produced at the cathode depends on the position of the metal in the reactivity series (below). N.B. Hydrogen and carbon are included for comparison.
- If the metal is more reactive than hydrogen, then hydrogen is produced.
- 2H+ (aq) + 2e- H2 (g)
- If the metal is less reactive than hydrogen, then the metal is produced.
- e.g. Cu2+ (aq) + 2e- Cu (s)
At the anode:
Oxygen is produced (from hydroxide ions), unless a halide ion is present (e.g. chloride, bromide, iodide).
- If no halide ion is present, oxygen is produced.
- 4OH- (aq) 2H2O (l) + O2 (g) + 4e-
- If a halide ion is present, then the corresponding halogen is produced.
- e.g. 2Cl- (aq) Cl2 (g) + 2e-
Back to Chemistry Home