
Structure & Bonding:
CONTENTS
CHEMICAL BONDING
DEFINITION: Element
An element is a chemical made of only one type of atom.
DEFINITION: Compound
A compound is two or more elements that are chemically joined.
Bonding happens because atoms try to achieve the electron configuration of noble gases (full outer shells). As such, atoms that do not have Noble Gas Electron Configurations are highly reactive.
For example, an oxygen atom has electron configuration [2,6]. To form a full outer shell of electrons, it must either get two more electrons, or lose 6 electrons. But, gaining 2 electrons is energetically favourable to losing 6 electrons, so oxygen atoms must gain 2 electrons to form a full outer shell.
Similarly, a magnesium atom has electron configuration [2,8,2] and therefore must lose 2 electrons to form a full outer shell of electrons.
There are three ways for atoms to achieve a full outer shell of electrons:
- They can gain electrons (to get more)
- They can lose electrons (to leave behind fewer)
- Or, they can share electrons (to have more)
ION FORMATION
DEFINITION: Ion
A charged particle that has lost or gained electrons.
When an atom gains or loses electrons, it forms what is called an ion. An ion is simply the name for a charged atom.
E.g. When a fluorine atom gains 1 electron it forms an ion with a charge of -1
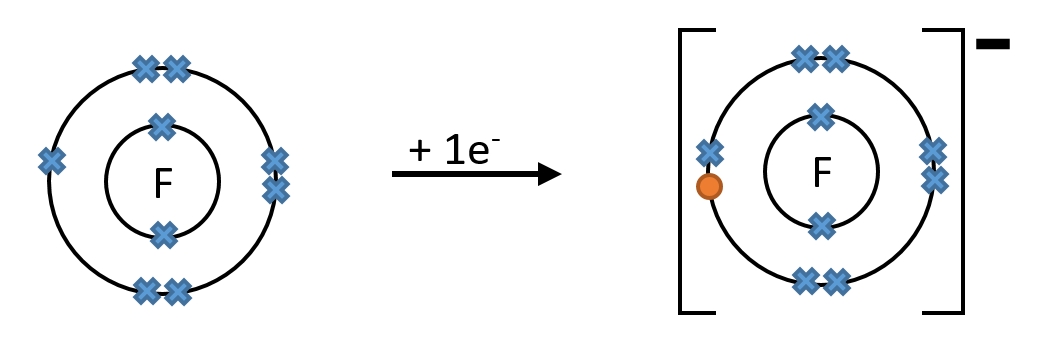
When a sodium atom loses 1 electron it forms an ion with a charge of +1
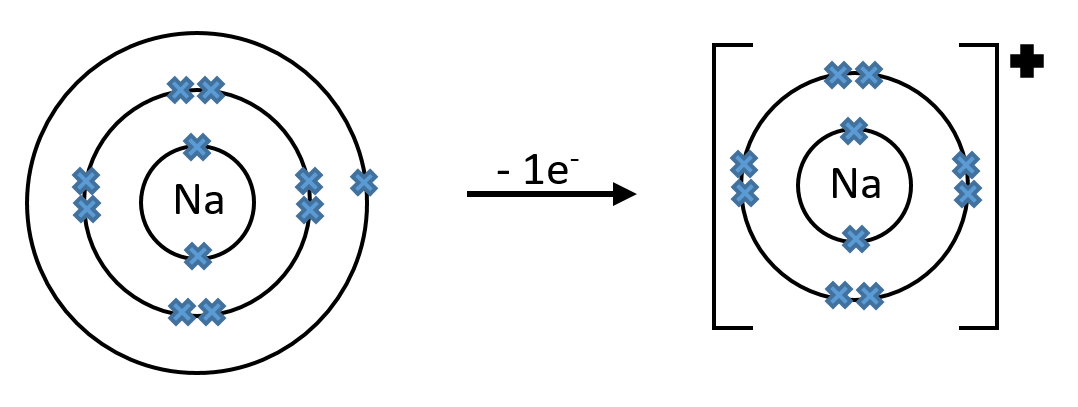
Note: In the above diagrams, all of the electrons are shown, however it is often easier to only draw the valence electrons (electrons in the outer shell).
Metal atoms form positive ions, and non-metal atoms form negative ions. You can find the charge of the ion formed by an atom using the periodic table. The number of charges on an ion formed by a metal is equal to the group number of the metal. The number of charges on an ion formed by a non-metal is equal to 8 minus the group number of the non-metal.
E.g. Magnesium is in group 2 and therefore forms an ion with a 2+ charge. Fluorine is in group 7 and therefore forms ions with a 1- charge.
Note: The noble gases (group 0/group 8) do not form ions as they already have a full outer shell of electrons.
Note: Carbon and silicon in group 4 generally form covalent bonds by sharing electrons (See Covalent Bonding).
IONIC BONDING
DEFINITION: Ionic Bonding
The electrostatic forces of attraction which hold positive and negative ions together in an ionic lattice.
These chemical interactions only occur between ions. Since opposite charges attract and like ones repel, the interaction must be between ions that are oppositely charged. Thus, any ionic compound must contain both positively and negatively charged ions.
For example, an ionic lattic can be formed between Na+ and Cl- ions; and can be formed between K+ and Cl- ions; but not between K+ and Na+ ions.
Since metals (from the left of the periodic table) form positive ions and non-metals (from the right of the periodic table) form negative ions, ionic compounds usually consist of one metal and one non-metal.
Transferring Electrons (gaining or losing):
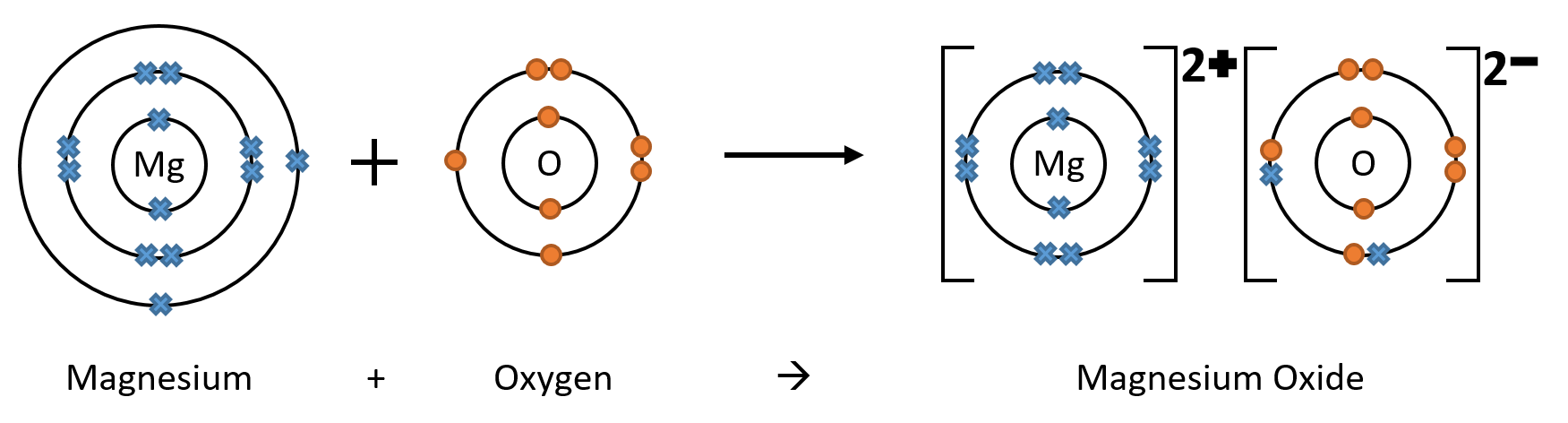
Electrons cannot be created or destroyed. So if an oxygen atom needs 2 electrons, it has to get them from somewhere. It may, for example, react with a magnesium atom to get the 2 electrons it wants. In this case, the simplest ratio of Mg to O is 1 to 1 (as oxygen ions have a charge of 2- and magnesium ions have a charge of 2+), so the compound takes the formula MgO.
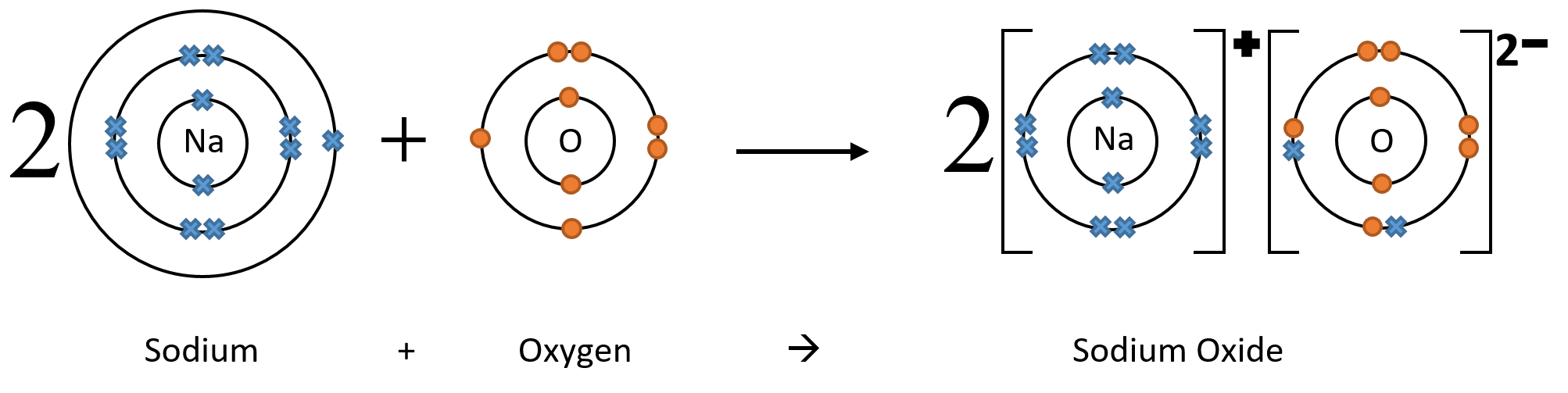
However, if an oxygen atom reacts with a sodium atom, it can only get 1 electron of the 2 it needs (as sodium ions only have a charge of +1). So the oxygen atom must react with 2 sodium atoms to get 2 electrons. In this case the compound contains 2 sodium ions for every 1 oxygen ion and has the formula Na2O
COVALENT BONDING
In addition to gaining or losing electrons to form full outer shells, atoms can share electrons.
DEFINITION: Covalent Bonding
The electrostatic forces of attraction between a shared pair of electrons and common nuclei.
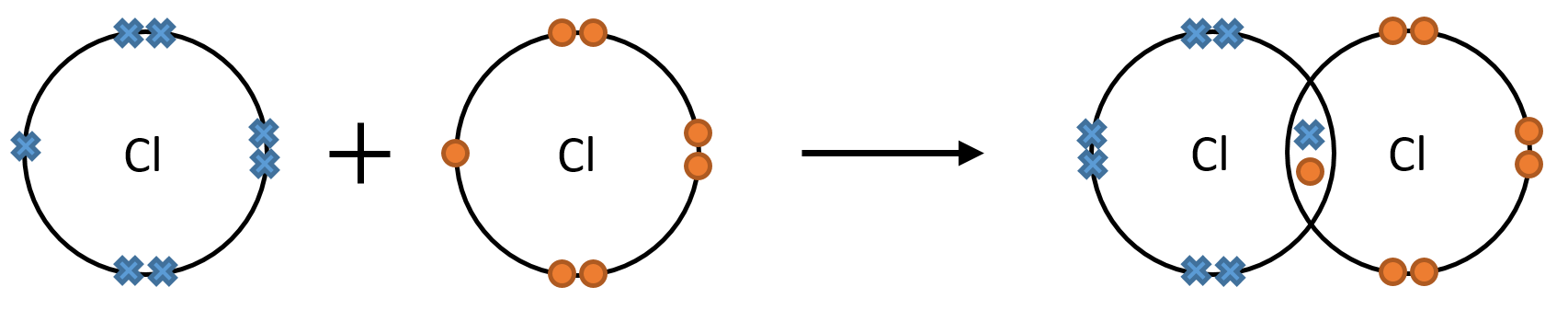
For example, chlorine atoms have seven electrons in their outer shell. They need one electron to form a full outer shell, so two chlorine atoms can do this by sharing one pair of electrons, where one electron comes to the pair from each atom.
Oxygen atoms need two electrons to form a full outer shell, so two oxygen atoms can achieve this by sharing two pairs electrons.
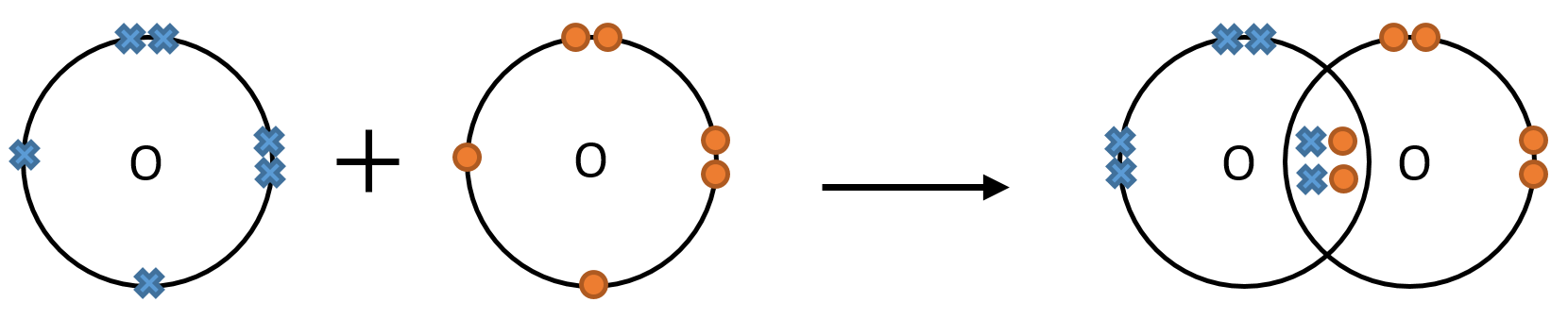
The name given to two atoms sharing a pair of electrons is a covalent bond and when two or more atoms have bonded covalently they are known together as a molecule
DEFINITION: Molecule
Two or more atoms bonded covalently.
The number of covalent bonds formed by an atom in any molecule is always the same for any element, and can be predicted from the position of an element in the periodic table. The number of covalent bonds formed by an element is 8 minus the group number of the element.
Note: Hydrogen is sometimes placed in group 1 of the periodic table, but it bonds covalently and always forms 1 covalent bond.
Only non-metallic elements form covalent bonds.
Bonding Between Different Atoms:
Previously, we have looked at examples of atoms of the same element bonding with each other. But, atoms can also form covalent bonds with atoms of different elements.
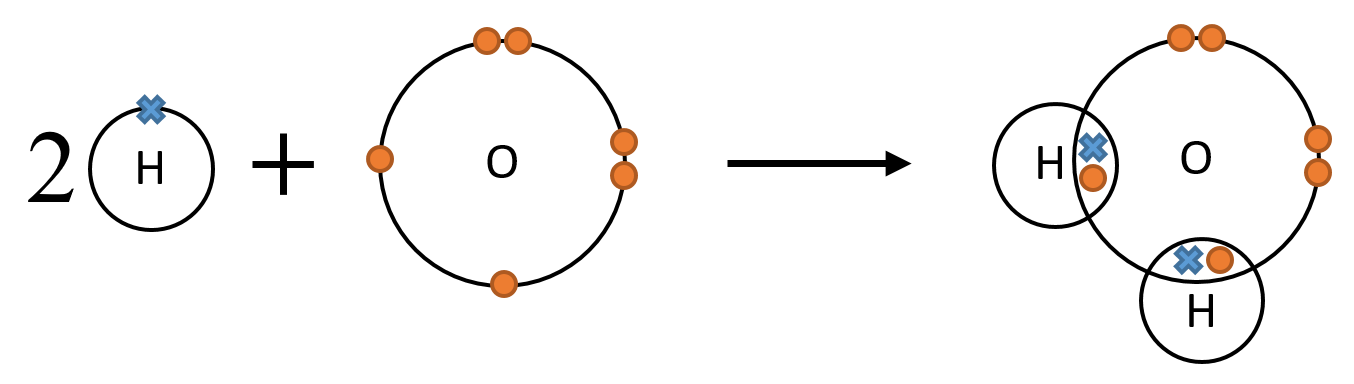
If one hydrogen atom covalently bonds with one oxygen atom, then the hydrogen atom forms a full outer shell, but the oxygen atom doesn't. An oxyen atom needs two electrons, so tries to share two pairs of electrons. It does this by sharing one pair with each of two hydrogen atoms, as shown below, forming water.
Covalent substances fall into two main categories:
- Simple molecules
- Giant covalent structures
Simple molecules are made up of a few atoms covalently bonded together. The covalent bonds that hold the atoms together are very strong, but the intermolecular forces between molecules are weak. Examples of simple molecules include water and carbon dioxide.
Giant covalent structures consist of many atoms, each joined to adjacent atoms by covalent bonds. They are usually arranged into a lattice structure, which is very strong due to the number of bonds involved. Examples of giant covalent structures include diamond and graphite, which are both allotropes of carbon.
If you want to learn more about simple molecules and giant covalent structures, see Structures and Properties
METALLIC BONDING
DEFINITION: Metallic Bonding
The electrostatic forces of attraction which hold positive ions and delocalised electrons together in a metallic lattice.
Only elements that are metals (from the left of the periodic table) can be present in metallic bonding. Atoms of metals always lose electrons, so always form positive ions.
Metals can lose their valence electrons (electons is the outer shell) even if there is nothing present to take them away. In this case, delocalised (free) electrons can float about between ions, as shown in the diagram below.
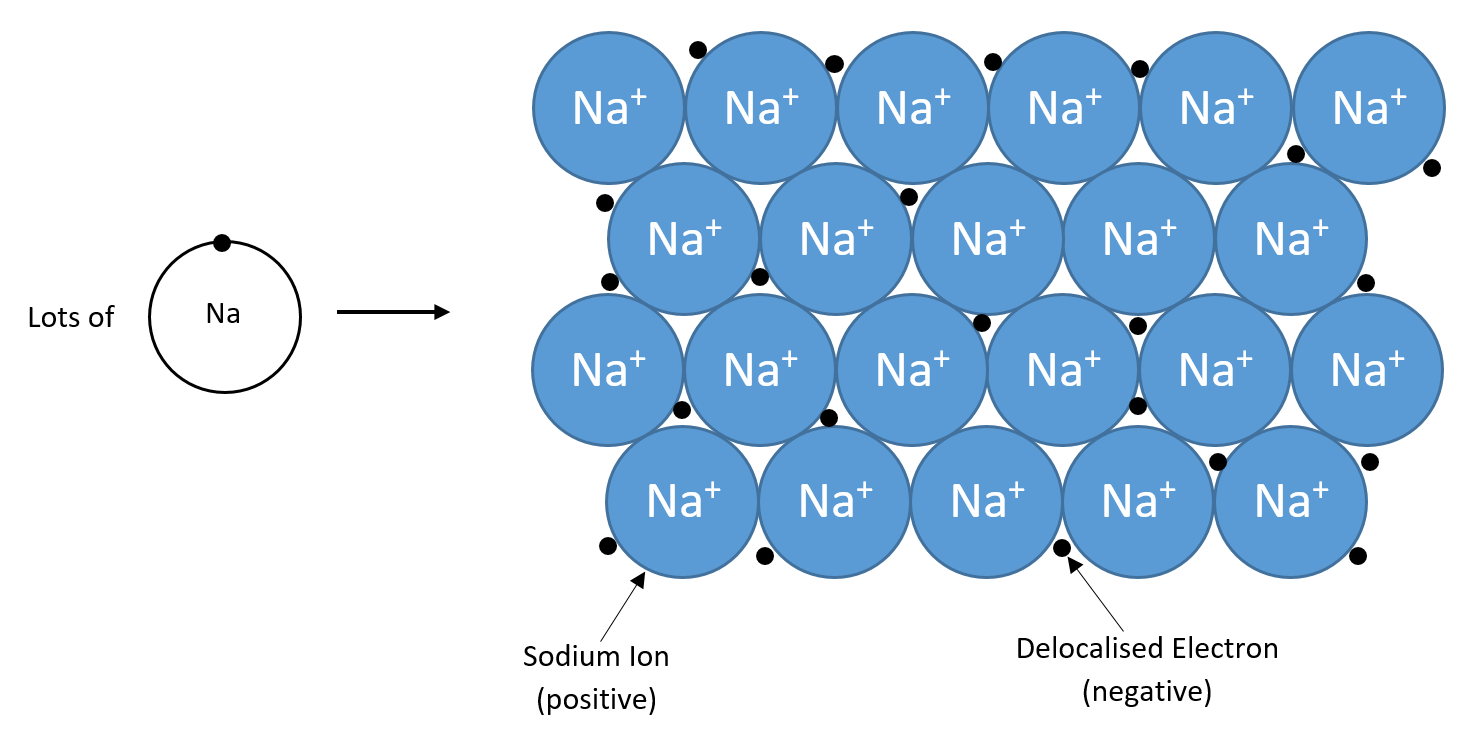
A metallic lattice is similar to an ionic one because it is held together by strong electrostatic forces of attraction, which take a large amount of energy to overcome. However, a metallic lattice is different because the forces act between positive ions and delocalised electrons. The diagram above shows the bonding in sodium. Positive sodium ions are held in place by their attraction to the delocalised (free) electrons.
STRUCTURES AND PROPERTIES
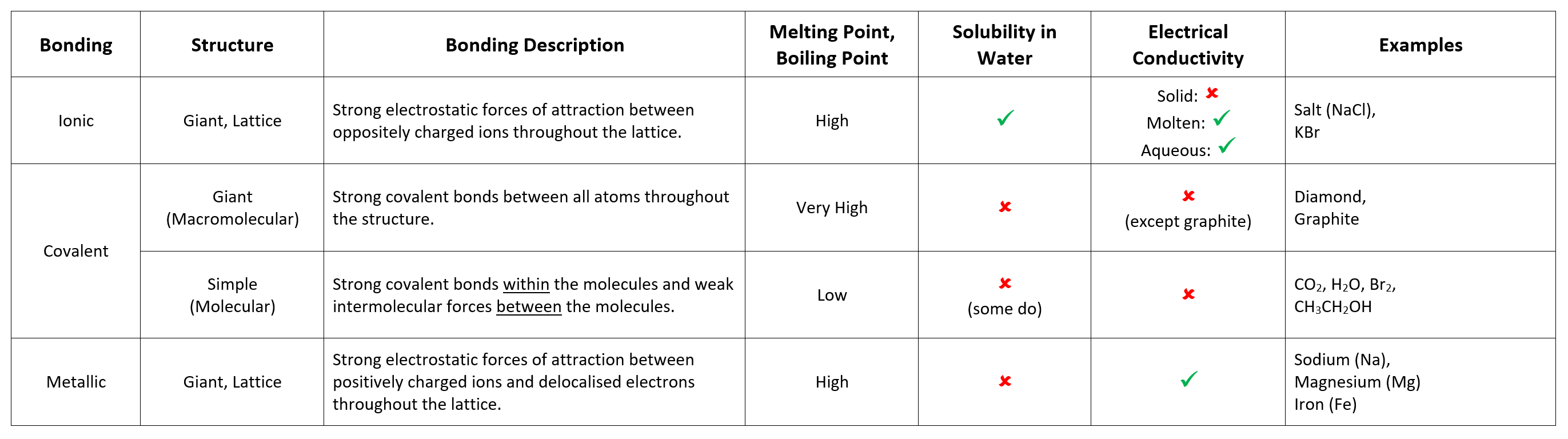
Ionic Properties:
- High Melting and Boiling Points: Ionic lattices are held together by strong electrostatic forces of attraction, which require large amounts of energy to be overcome.
- Good Conductors (as liquids) Ionic lattices contain many ions. When molten (melted), the ions can move and carry charge.
- Bad Conductors (as solids) In a solid ionic compound, ions are not free to move, so no charge can flow through it.
- Very Good Solubility in Water: Water is able to attract ions.
Simple Covalent Properties:
- Low Melting and Boiling Points: Simple covalent substances have weak intermolecular forces between the molecules, which require little energy to break.
- Do Not Conduct Electricity: Simple covalent substances do not have any free electrons, or an overall electric charge.
Giant Covalent Properties:
- Very High Melting and Boiling Points: Giant covalent substances have strong covalent forces between the molecules, which require large amounts of energy to break.
- Most Do Not Conduct Electricity: Most giant covalent substances do not have charged particles that are free to move, meaning most cannot conduct electricity. Graphite, a form of carbon that can conduct electricity, is an exception.
Metallic Properties:
- High Melting and Boiling Points: Metallic lattices are held together by strong electrostatic forces of attraction, which require large amounts of energy to be overcome.
- Good Conductors of Electricity: Metallic lattices contain many delocalised electrons, which carry charge through the metal.
- Metals are Ductile and Malleable: Metallic lattices are layered structures; these layers of cations can slide over each other, allowing the metal to be easily shaped or dented.
SUMMARY
Back to Chemistry Home